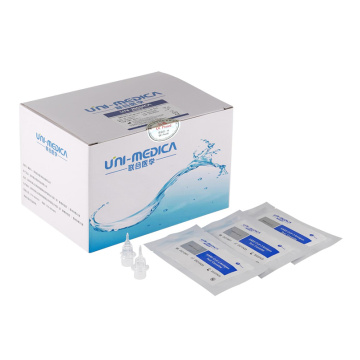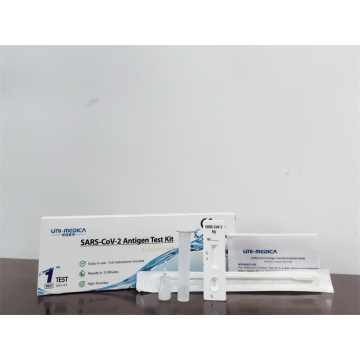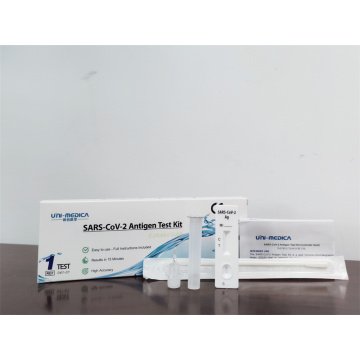
TGA SARS-CoV-2 antigen rapid test kit
- Min. Order:
- 10000 Piece/Pieces
- Min. Order:
- 10000 Piece/Pieces
- Transportation:
- Ocean, Land, Air, Express
- Port:
- Shenzhen, Guangzhou, Hongkong
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | China |
|---|---|
| Productivity: | 1,000,000,000 pcs/month |
| Supply Ability: | 1,000,000,000,000 pcs/year |
| Payment Type: | L/C,T/T,D/P,D/A,Paypal,Cash |
| Incoterm: | FOB,CFR,CIF,EXW,FAS,Express Delivery,DAF,DDU,DDP |
| Certificate: | CE/SGS |
| HS Code: | 3822190020 |
| Transportation: | Ocean,Land,Air,Express |
| Port: | Shenzhen,Guangzhou,Hongkong |
Product Name
COVID-19 Rapid Antigen Detection Kit
Alternative Name
SARS-CoV-2 Antigen Rapid Test Kit, also named Covid-19 Antigen Test Kit, SARS-CoV-2 Antigen Kit, etc.
Intended Use
The Novel Coronavirus (SARS-CoV-2) Antigen Rapid Test Kit is a chromatographic immunoassay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal swab, oropharyngeal swab,saliva or nasal swab specimens directly from individuals who are suspected of COVID-19 by their healthcare provider within the first 7 days of symptom onset.
It only recognizes the N protein and cannot detect the S protein and its mutation structure.
The test provides preliminary test results. Negative results do not preclude SARS-CoV-2 infection and they cannot be used as the sole basis for treatment or other management decision.
Product Description
This SARS-CoV-2 Rapid Antigen Detection Kit(Colloidal Gold) uses the sandwich immunocapture method and colloidal gold immunochromatography to qualitatively determine the presence of SARS-CoV-2 antigens in human oropharyngeal swabs, nasal swabs and nasopharyngeal swabs. It is helpful as an aid in the screening of early mild, asymptomatic, or acute patients for identification of SARS-CoV-2 infection.
Detection Principle
This Covid-19 Antigen Test Kit uses immunochromatography.
Scope of Use
It is used for in vitro qualitative detection of novel coronavirus antigens in nasopharyngeal swabs and oropharyngeal swabs to diagnose diseases caused by novel coronavirus infections.
It is suitable for the rapid investigation of suspected cases of large-scale new crown virus infection, and is very effective for rapid diagnosis of concentrated outbreaks, thereby promoting early diagnosis and timely intervention of patients, which can better meet the needs of rapid detection and prevention on the spot of epidemics in various countries, and help on a global scale Prevention and control of the new coronavirus epidemic.
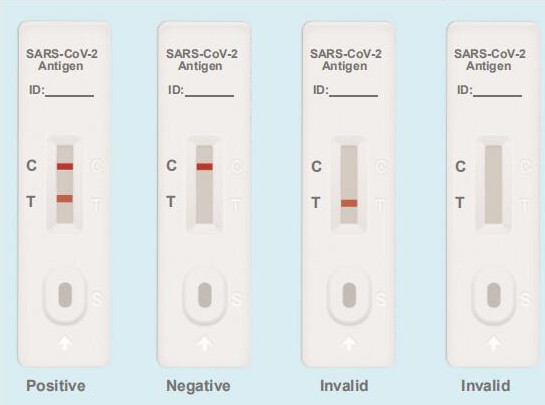
Testing Results
Positive: Both the detection line (T line) and the quality control line (C line) appear colors.
Negative: The test line (T line) does not appear color, only the quality control line (C line) appears color
Invalid: The quality control line (C line) does not appear color, or both of the quality control line (C line) and the test line (T line) do not appear color, which means that the test is invalid and the test should be repeated.
Our advantage
1. Quick result: < 5 minunites
2. High accuracy: >99%
3. High sensitivity:>99%
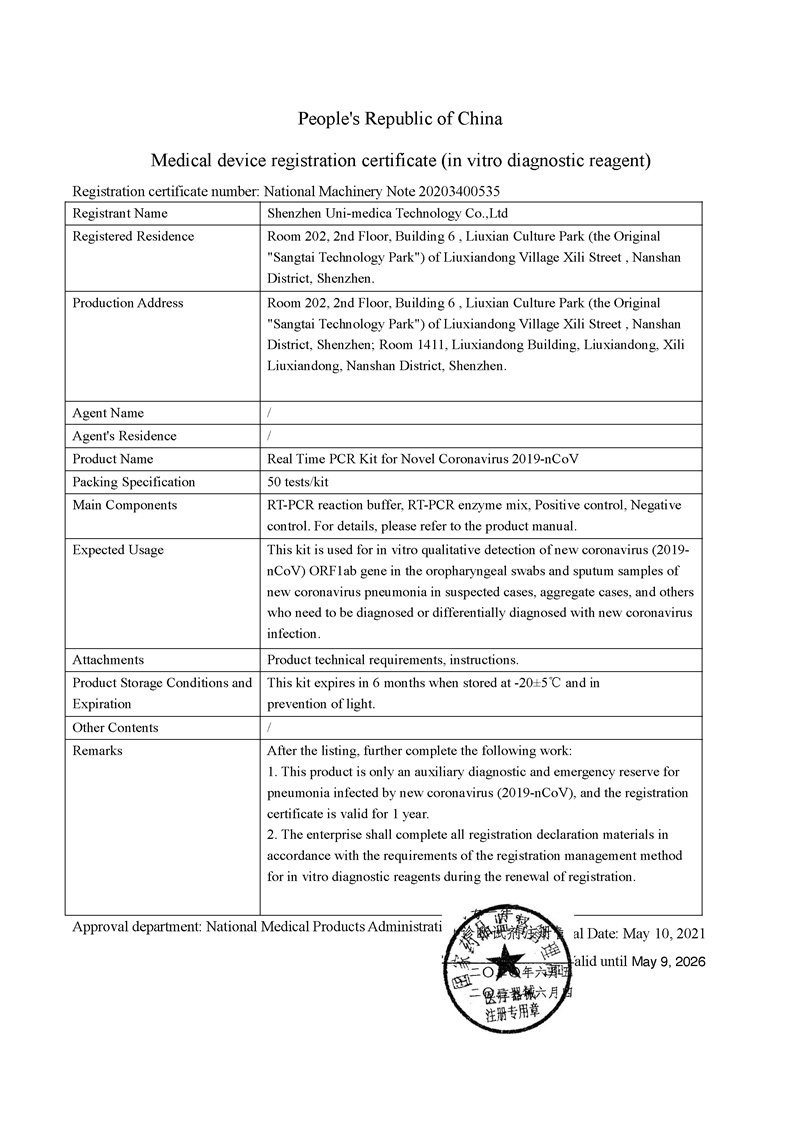
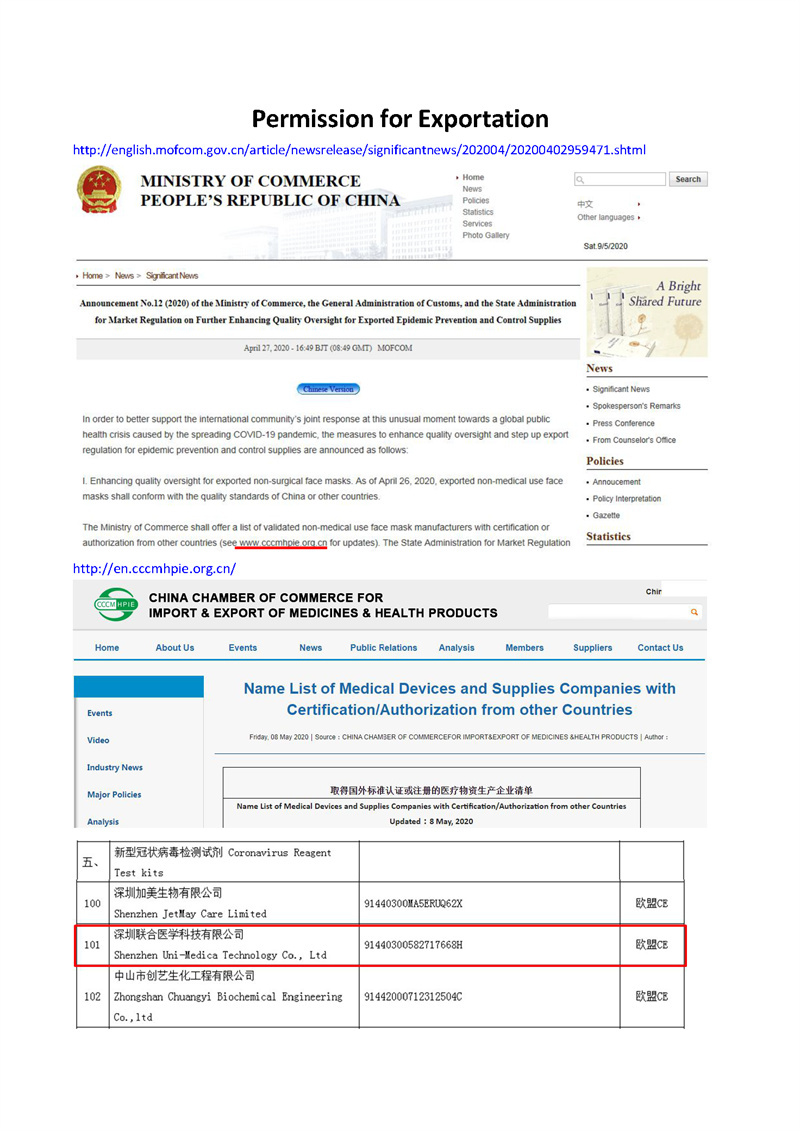
This SARS-CoV-2 antigen rapid test kit has been under application of TGA approval. It will be TGA approved in one month.
If you are interested in promoting this Covid-19 antigen rapid test kit in Australia, welcome to get samples.


Related Keywords