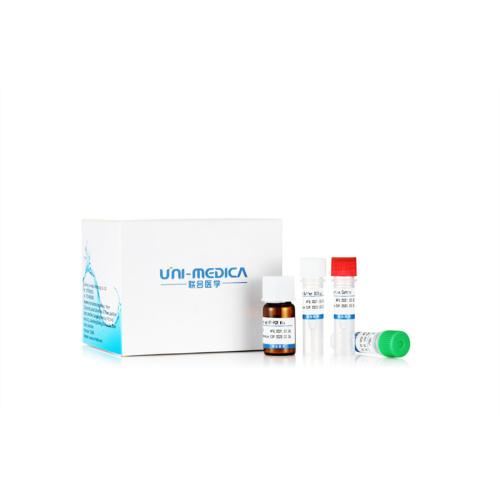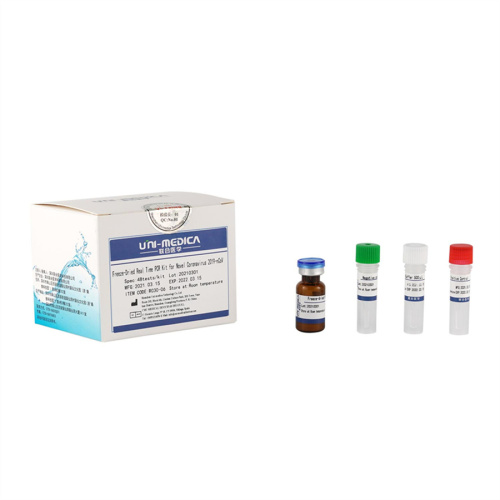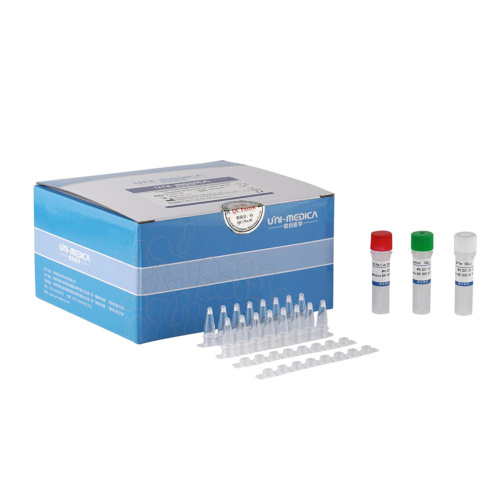
Freeze-dried Novel Coronavirus Test Reagent(ORF1ab,N)
- Min. Order:
- 10000 test
- Min. Order:
- 10000 test
- Transportation:
- Ocean, Land, Air, Express
- Port:
- Shenzhen
Quantity:
Your message must be between 20 to 2000 characters
Contact NowBasic Info
Basic Info
| Place of Origin: | Shenzhen,China |
|---|---|
| Productivity: | 1 000 000 000 reactions/month |
| Supply Ability: | 10 000 000 000 reactions/year |
| Payment Type: | L/C,T/T,D/P,D/A |
| Incoterm: | FOB,CFR,CIF,EXW |
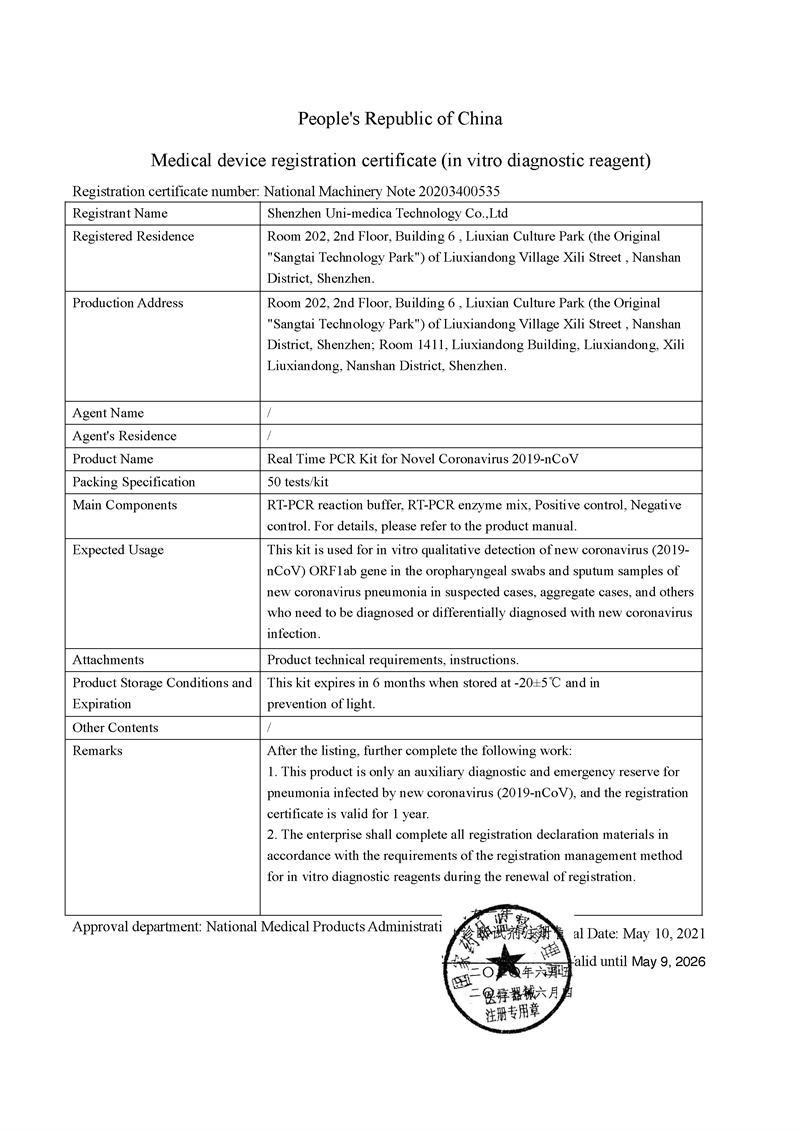
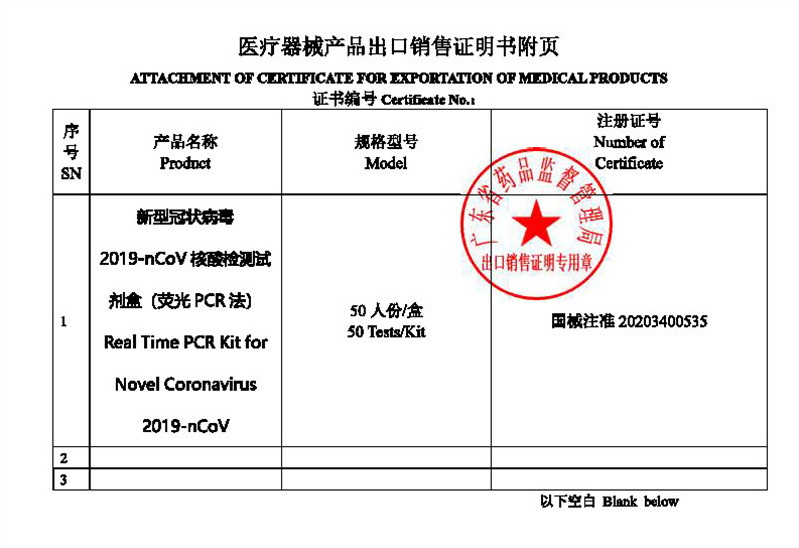
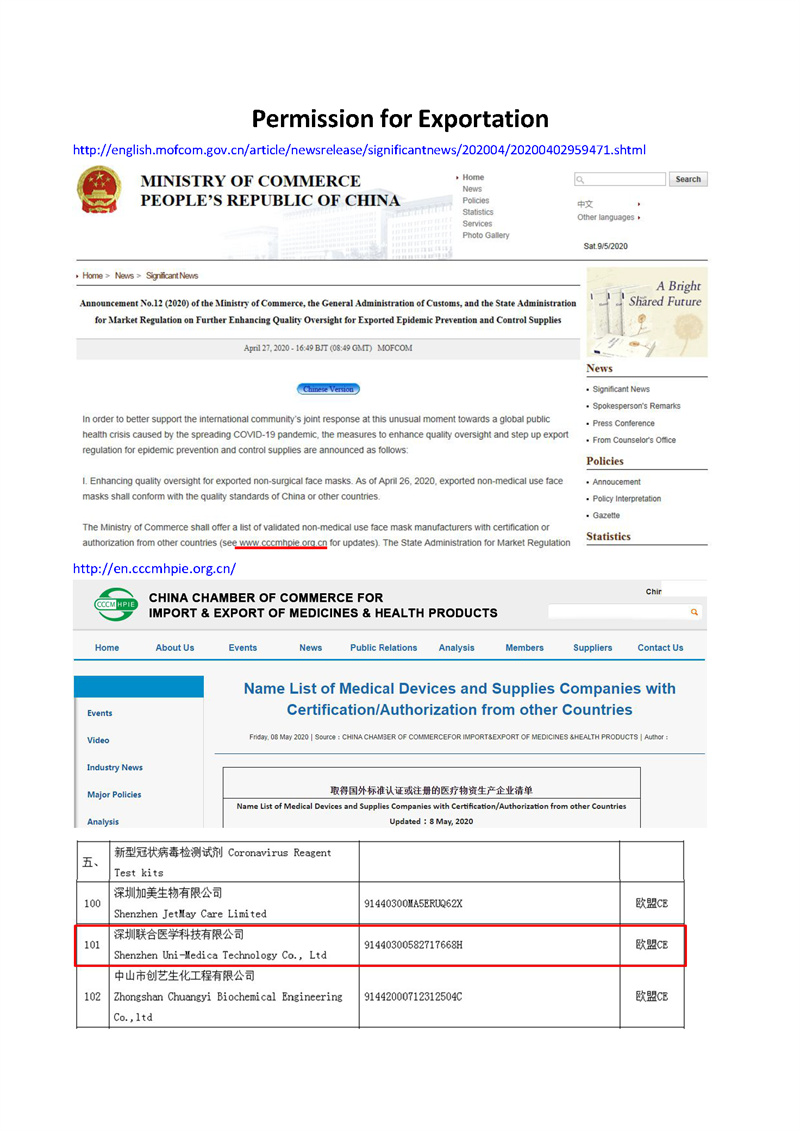
| Certificate: | CE/SGS |
| HS Code: | 3822009020 |
| Transportation: | Ocean,Land,Air,Express |
| Port: | Shenzhen |
Product Description
Product Description
[Product Name]
Freeze-dried Novel Coronavirus Test ReagentFreeze-Dried Real Time PCR Kit for Novel Coronavirus 2019-nCoV
[Alternative Name]
Novel Coronavirus Test Reagent, also named Freeze-dried Pcr Test Reagent, etc.
[Product Description]
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be contagious. Based on the current
epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
This Novel Coronavirus Detection Reagent kit is used for in vitro qualitative detection of novel coronavirus 2019-nCoV in respiratory specimens including oropharyngeal swab, sputum, bronchoalveolar lavage fluid and nasopharyngeal swab. Primer sets and FAM labeled probe are designed for specific detection of ORFlab gene of 2019-nCoV, VIC labeled probe for N gene of 2019-nCoV. Human RNase Pgene extracted concurrently with the test sample provides an internal
control to validate nucleic extraction procedure and reagent integrity. Probe targeting human RNase P gene is labeled with CY5.
[Storage and Expiry date]
1.This Novel Coronavirus Testing Reagent kit should be stored at room temperature and in prevention of light and expires in 12 months.
2.The Freeze-Dried reagents is vacuum-packed and sealed in an aluminum foil bag. After opening, please store the unused products in the provided resealable plastic bag with the desiccants, squeeze out the air, and put back in the aluminum foil bag. The Positive Control should be kept at -20 ± 5 ℃ after reconstitution.
3.Manufacture date and expiration date are printed on the label.
[Applicable instrument]
Real-Time PCR System: Molarray MA-6000, ABI 7500, ViiATM 7, Quant Studio7flex, Roche Lightcycler 480, Agilent Mx3000P/3005P, Rotor-GeneTM 6000/Q, Bio-Rad CFX96 TouchTM/iQTM 5,SLAN-96S/96P, etc.
[Our Advantage]
1. High sensitivity: 100-200 copies/ml.
2. Short amplification time: 52 mins
3. Quick delivery time: 1-3 days.




Related Keywords
Related Keywords