Medical Viral Collection Transport Medium Tube
- Min. Order:
- 10000 Piece/Pieces
- Min. Order:
- 10000 Piece/Pieces
- Transportation:
- Ocean, Land, Air, Express
- Port:
- Shenzhen
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | China |
|---|---|
| Productivity: | 10 000 000 tests/month |
| Supply Ability: | 10 000 000 tests/month |
| Payment Type: | L/C,T/T |
| Incoterm: | FOB,CFR,CIF,EXW |
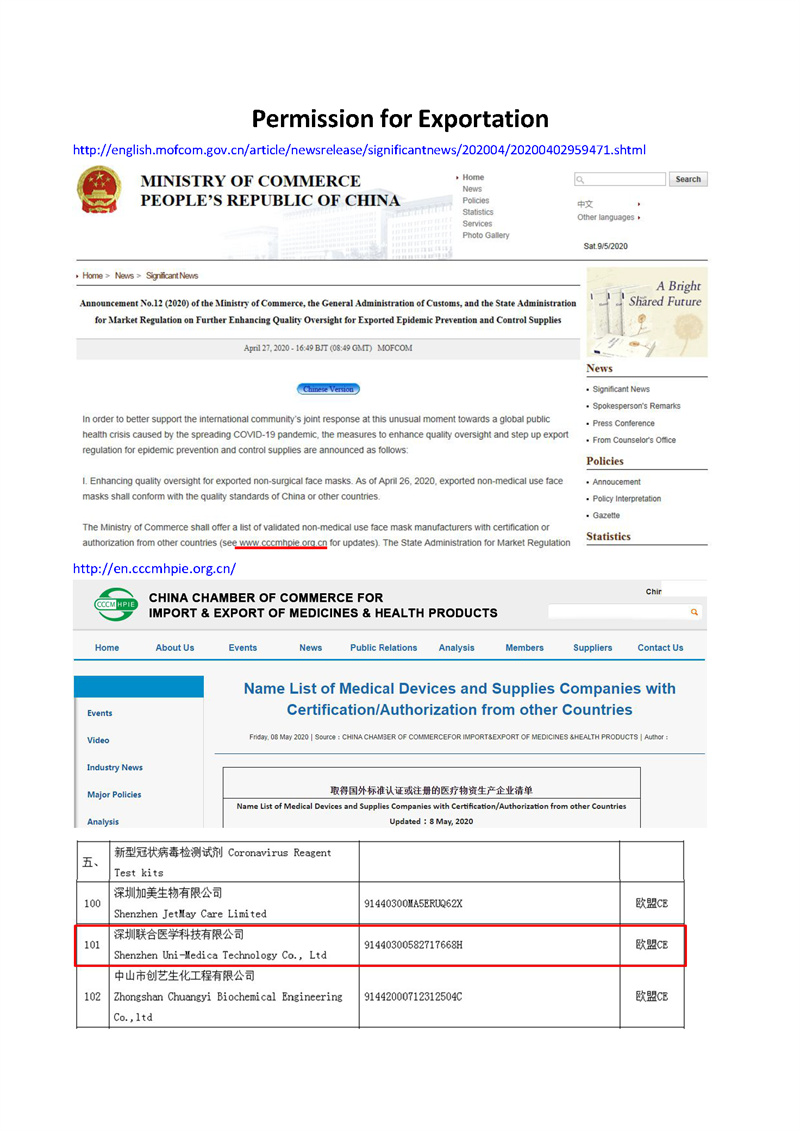
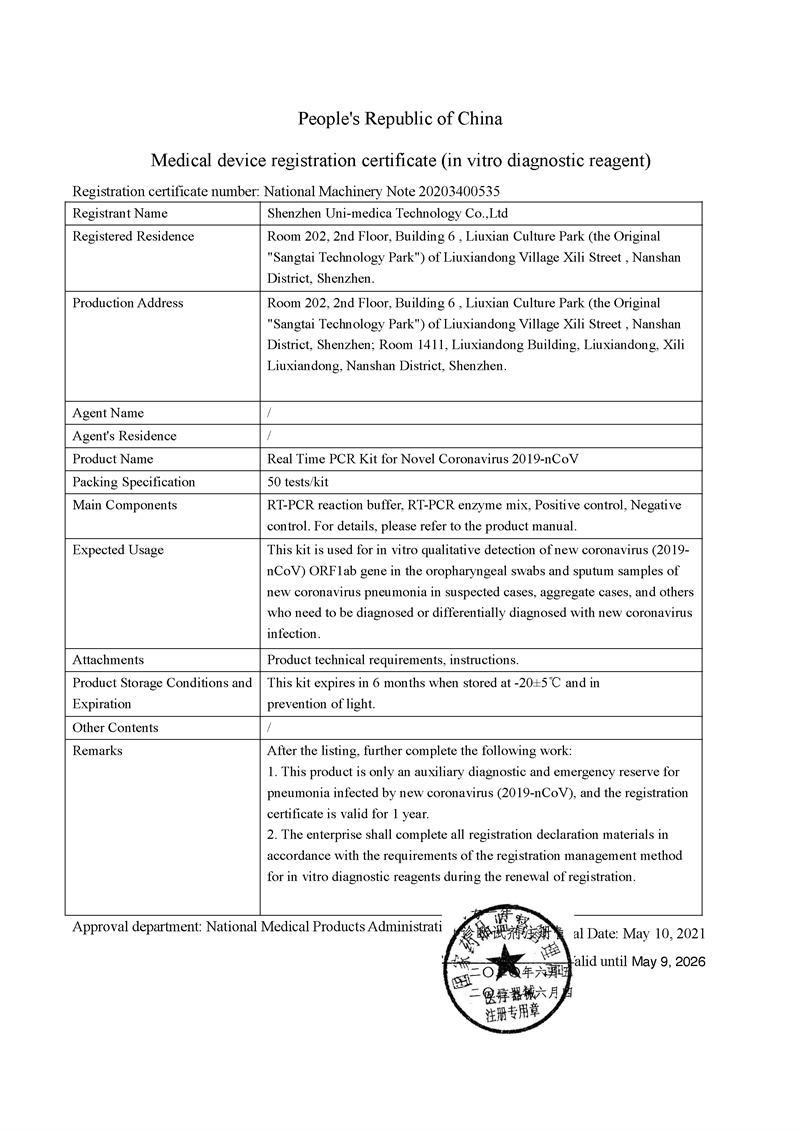
| Certificate: | CE/SGS |
| HS Code: | 3821000000 |
| Transportation: | Ocean,Land,Air,Express |
| Port: | Shenzhen |
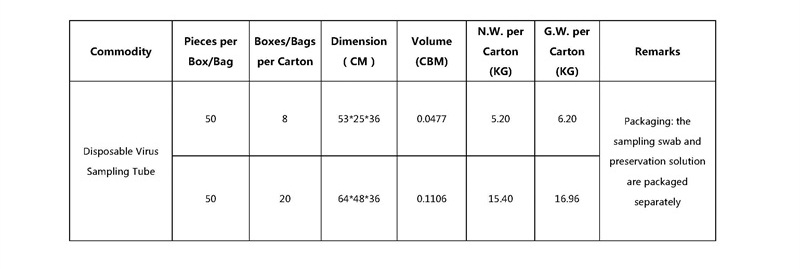
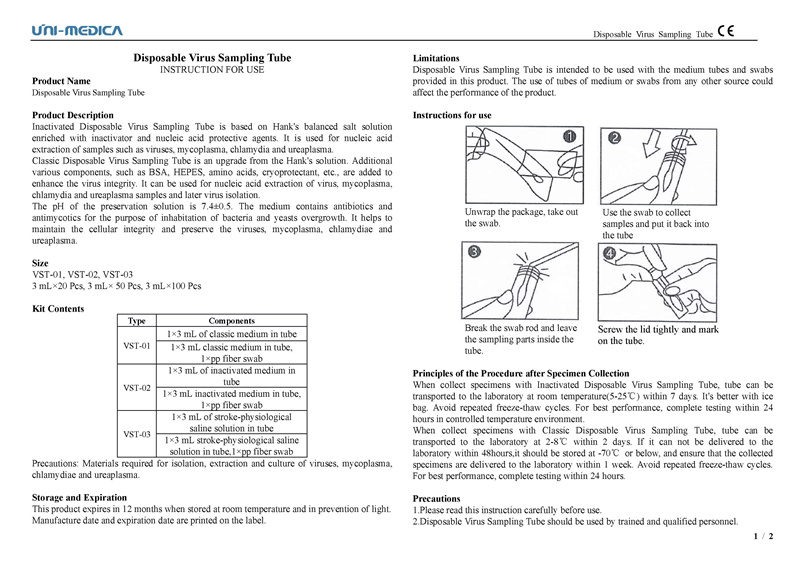
The Disposable Virus Sampling Tube consists of a PP fiber swab and an inactivated medium, which has the effect of a virus inactivating agent, inactivating the virus sample and protecting RNA from degradation, effectively reducing the risk of aerosol contamination. The thickening of the sample tube and the design of leakage prevention ensure that the sample does not leak and comply with WHO regulations and biosafety regulations.
Product Name
Inactivated Disposable Virus Sampling Tube
Product Description
This Inactivated Disposable Virus Sampling Tube is a Disposable Virus Sampling Tube.
The virus preservation solution added with lysis salt is an inactivated virus preservation solution. The main purpose of the Tris, lysis salt, EDTA, etc. contained in it, is to lyse the nucleic acid and release the nucleic acid, so that the nucleic acid can be detected by the subsequent real-time fluorescent RT-PCR. This is to determine whether the sample contains virus characteristic nucleic acid, that is, whether it is infected with the virus.
Nucleic acid is a biological macromolecular compound formed by the polymerization of many nucleotides, including deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), and is one of the most basic substances of life. The virus has a single structure and contains only one type of nucleic acid and protein. Therefore, the virus will be detected if the characteristic nucleic acid is detected. Viruses are parasitic organisms and cannot survive outside the body after sampling. If they cannot be detected in time, they need to be placed in the virus preservation solution. In order to protect the safety of the virus detection environment, it is necessary to add lysis salt to inactivate the virus and release the nucleic acid that can be detected.
Features of single-use virus sampling tube (inactivated type)
1. Simple operation and use, no need to prepare liquid, the system contains high-efficiency virus lysate, the virus is inactivated immediately after sampling, effectively preventing the risk of secondary infection, and ensuring the safety of transportation and testing personnel;
2. Viral DNA/RNA can be stored and transported at room temperature without degradation for 1 week. After extraction according to most commercial kits, the DNA/RNA obtained is of good quality and high yield, and can complete various genetic testing and analysis experiments. Such as PCR, qPCR, etc., while saving transportation costs;
3. Contains nucleic acid RNase inhibitors to maximize the protection of viral nucleic acids from being degraded and greatly improve the efficiency of nucleic acid extraction.
4. This Inactivated VTM is suitable for the collection, preservation and transportation of common virus samples such as new coronavirus, influenza virus, hand, foot and mouth virus. This Virus Sampling Tube can collect throat swabs and nasal test samples or tissue samples of specific parts. The stored samples can be used for subsequent clinical trials such as nucleic acid extraction or purification.
【Precautions】
1. It is forbidden to directly contact the person being collected with the preservation solution that has been placed in the virus sample.
2. It is forbidden to sample the swab after soaking the swab with the preservation solution.
3. This Inactivated Disposable Virus Tube is a disposable product and is only used for the collection, transportation and storage of clinically collected virus specimens. It should not be used beyond the intended use.
4. The product must not be used after the expiry date or the product packaging is damaged.
5. When collecting virus samples, professionals should strictly follow the sampling procedures; when samples are tested, they should be operated in a laboratory that meets the safety level.
【Our advantage】
1. Accurate test results: Use the best guanidine salt concentration to ensure the accuracy of the test results.
2. Stable performance: unchanged performance within one year
3. Short delivery time: normally 1-3 days






Related Keywords