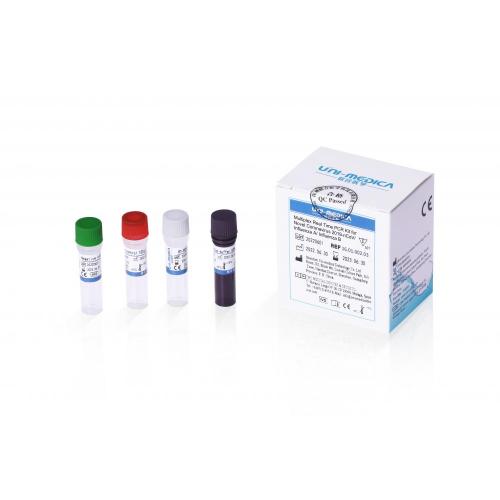
Multiplex Real Time PCR Kit for Novel Coronavirus 2019-nCoV/Influenza A/Influenza B
- Min. Order:
- 5000 T
- Min. Order:
- 5000 T
- Transportation:
- Ocean, Air, Land
- Port:
- Shenzhen , Guangzhou, Hong Kong
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | China |
|---|---|
| Productivity: | 100,000,000T/year |
| Supply Ability: | 10,000,000T/month |
| Payment Type: | L/C,T/T |
| Incoterm: | EXW,FOB,CFR,CIF,FCA |
| Certificate: | CE, ISO13485, ISO9001 |
| HS Code: | 3822009000 |
| Transportation: | Ocean,Air,Land |
| Port: | Shenzhen ,Guangzhou,Hong Kong |
Product name:
RT PCR detection reagent kit for 2019-nCov, Influ A & B
Intend use:
This RT PCR detection reagent kit for 2019-nCov, Influ A & B is used for in vitro qualitative detection of 2019-Novel Coronavirus (2019-nCoV), Influenza A and B in respiratory specimens including oropharyngeal swabs and sputum. Primer set and FAM labeled probe are designed for specific detection of ORFlab gene of 2019-nCoV, VIC labeled for human RNase P gene as internal control, ROX labeled for M gene of Influenza A, CY5 labeled NP gene of Influenza B.
Features:
The RT PCR test reagent kit for 2019-nCov, Influ A & B, is a one -tube Multiplex PCR reaction for identification and detection of Covid-19, influenza A and B. Regarding to this real-time PCR detection kit , its Primer and Probe mix based on multi-target gene design the confirmatory gene ORF 1ab gene for the detection of 2019 novel coronavirus, and influenza A and B in human sample.
The Real-time PCR test reagent kit for 2019-nCov, Influenza A & Influenza B is highly compatible with Open ended RT PCR instruments with FAM, HEX/VIC, RED/ROX , and CY5 channels. Shenzhen Uni-medica RT-PCR Kit is compatible with popular Molarray MA-6000, ABI 7500, ViiATM 7, QuantStudio 5, QuantStudio 6/7 pro, QuantStudio 6/7 flex, Agilent Mx3000P/3005P, Rotor-GeneTM 6000/Q, Bio-Rad CFX96 TouchTM/iQTM 5,Hongshi SLAN-96S/96P, Roche Light Cycler 480, Molarray MA-1630Q, AGS8830, AGS4800 and other PCR instruments with FAM, HEX/VIC, RED/ROX , and CY5 channel.
Shenzhen Uni-medica RT PCR test reagent kit has been approved by the state drug administration and obtained China FDA and CE certification. Shenzhen Uni-medica PCR detection kit has been verified by 600+ clinical samples and used in more than 100 hospitals and Centers for Disease Control and Prevention at home and abroad.
Advantages:
1.One-tube one-step reaction
2.Hihg sensitivity: LOD is 200copies/ml
3.Short reaction time:52 mins on rapid PCR.
4.The whole process is monitored by internal control
5. Excellent performance but low price6.Short leading time : 1- 3 days
Shenzhen Uni-medica Technology Co., Ltd (Uni-medica) is a national high-tech enterprise. It has established cooperation and jointly performed a number of scientific research projects with the Hong Kong University of Science and Technology, Shenzhen University and Chinese Academy of Sciences. Uni-medica was also selected as one of the top 100 new Chinese innovative enterprises. The company takes the technical innovation of molecular detection methods as the core driving force, and focuses on the R&D and industrialization of molecular diagnostic products aiming at prevention and control of infectious diseases and genetic diseases. Uni-medica provides total solution of accurate diagnosis of infectious diseases and genetic diseases based on multi-omics technologies, including multiplex PCR, NGS technology and flow cytometry. Uni-medica is qualified by ISO 13485 and NMPA quality control system. The pathogen molecular detection products are daily used in hundreds of hospitals, Centers for Disease Control and Prevention and Quarantine Laboratories throughout the country, providing strong support for prevention and control of infectious diseases.





Related Keywords