96T DNA RNA extraction kit
- Min. Order:
- 10000 Others
- Min. Order:
- 10000 Others
- Transportation:
- Ocean, Land, Air
- Port:
- Shenzhen, Guangzhou, Hong Kong
Your message must be between 20 to 2000 characters
Contact Now| Place of Origin: | China |
|---|---|
| Productivity: | 10,0000,000T/month |
| Supply Ability: | 10,000,000T/month |
| Payment Type: | L/C,T/T |
| Incoterm: | FOB,CFR,CIF,EXW,FCA |
| Certificate: | CE, FDA |
| HS Code: | 3822009000 |
| Transportation: | Ocean,Land,Air |
| Port: | Shenzhen,Guangzhou,Hong Kong |
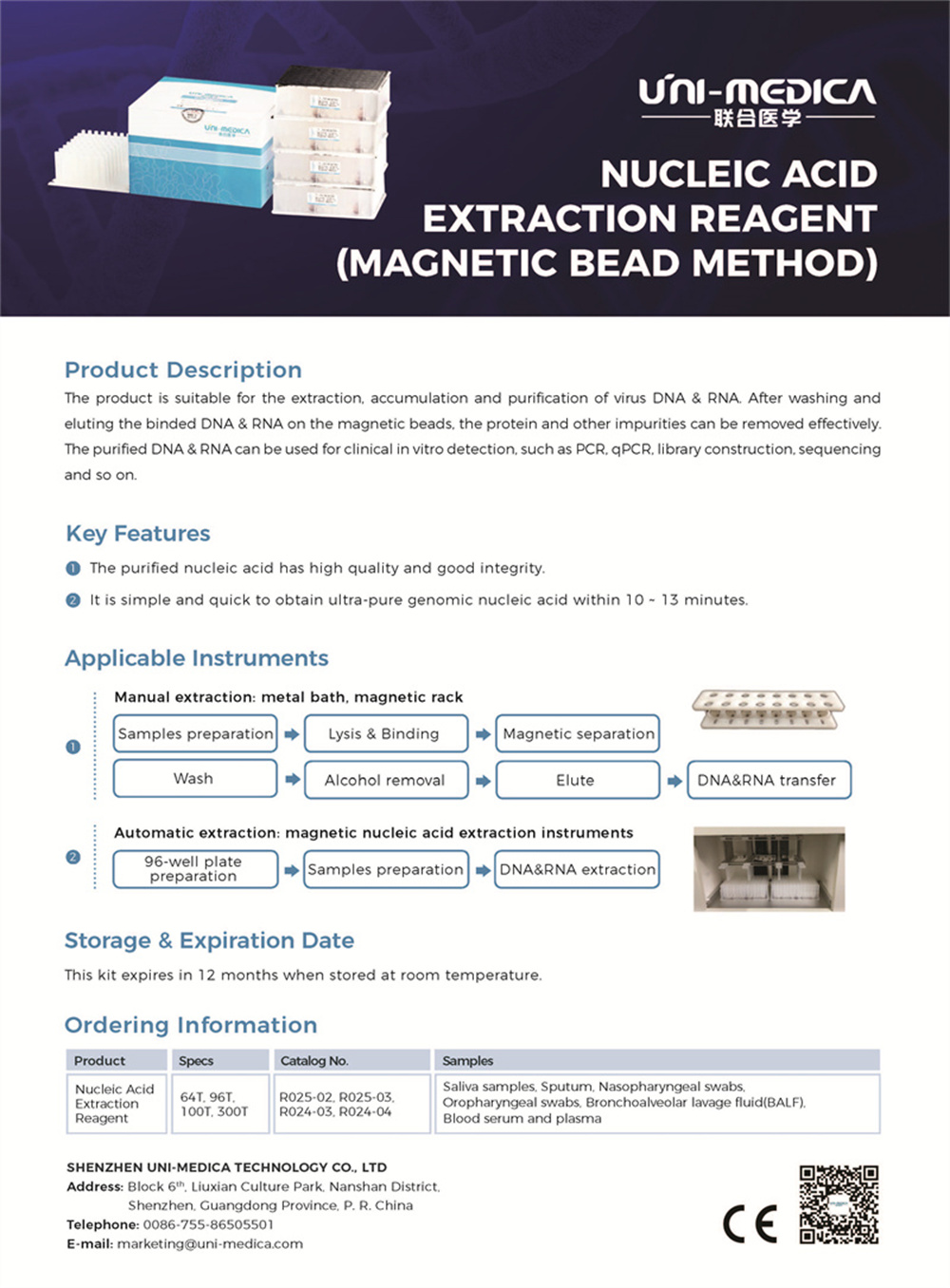
Shenzhen Uni-medica Nuleic Acid Extraction Reagent kit is developed based on magnetic bead technology. This DNA RNA extraction reagent kit is suitable for virus nucleic acid isolation, nucleic acid purification,and accumulation of oropharyngeal swabs, nasopharyngeal swabs, saliva, serum, plasma, etc., for downstream PCR detection. All the nucleic acid extracting reagent and magnetic beads are preloaded in 96-deep–well plate.
Shenzhen Uni-medica Nucleic Acid Purification Reagent kit shows not only easy and convenient operation, but also reliable and stable performance. Shenzhen Uni-medica Nucleic Acid Purification Reagent kit has been widely exported and used in more than 100 hospitals at home and abroad.
Shenzhen Uni-medica Technology Co., Ltd (Uni-medica) is a national high-tech enterprise. It has established cooperation and jointly performed a number of scientific research projects with the Hong Kong University of Science and Technology, Shenzhen University and Chinese Academy of Sciences. Uni-medica was also selected as one of the top 100 new Chinese innovative enterprises. The company takes the technical innovation of molecular detection methods as the core driving force, and focuses on the R&D and industrialization of molecular diagnostic products aiming at prevention and control of infectious diseases and genetic diseases. Uni-medica provides total solution of accurate diagnosis of infectious diseases and genetic diseases based on multi-omics technologies, including multiplex PCR, NGS technology and flow cytometry. Uni-medica is qualified by ISO 13485 and NMPA quality control system. The pathogen molecular detection products are daily used in hundreds of hospitals, Centers for Disease Control and Prevention and Quarantine Laboratories throughout the country, providing strong support for prevention and control of infectious diseases.





Related Keywords